Radioactive Radium
What is Radium?
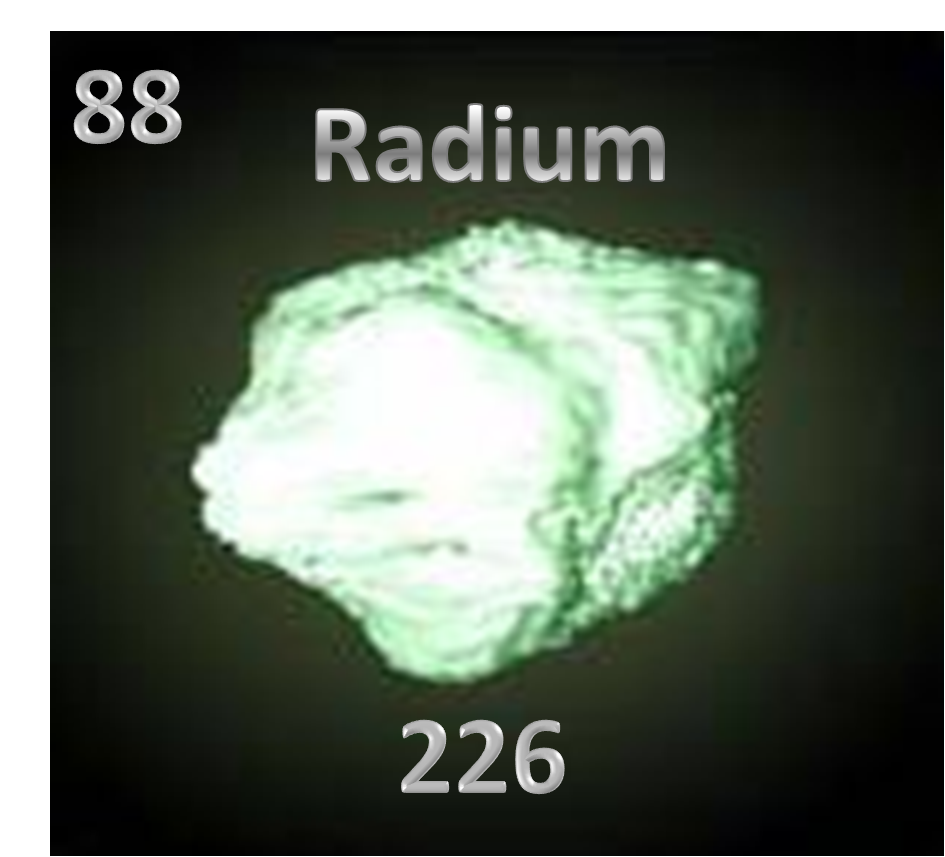 |
Fig.0- Radium
|
Radium(Fig.0) is the 88th element on the periodic table (Fig.1). The elemental symbol for radium is Ra (Fig.2). It’s one of the most reactive and rare of the earth alkaline earth metal, which is the sixth element in group 2 of the periodic table. It has a total mass of 226 a.m.u, which is fairly heavy. Radium came from the Latin word radius, meaning ray, cool, eh?
 |
| Fig.2- Atomic number |
 |
| Fig.3- Element symbol for radium |
Discovery
 |
| Fig.3- Marie and Pierre Curie |
 |
| Fig.4- Pitchblende |
Radium was discovered in 1898 by Pierre and Marie Curie (Fig.3). Marie Curie, who was a polish chemist and Pierre, who was a french chemist, obtained 1 mg of radium from a pitchblende (Fig.4), which is a material that contained uranium, so then Marie had to get more pitchblendes, to obtain more radium because 1 pitchblende only contained little radium. But! After noticing that the unrefined pitchblende was far more reactive than the uranium, they both were separated. Marie Curie eventually died from working with radium. She developed leukemia and died in 1934.
What is Radium used for?
 |
| Fig.5- Radium used in toothpastes |
 |
| Fig.6- Experiments on radium |
Before the dangers of radium were noticed, it was used in consumer products, which ranged from toothpastes to elixirs (Fig.5). Nowadays, due to many conducted experiments on radium (Fig.6), it is no longer used in household products. Radium as also been used in signs and illuminate watches, due to it’s ability to glow in the dark (Fig.7). Now, this one’s cool, did you know that radium was used to produce radon, which is a form of a radioactive gas to help treat cancer!
 |
| Fig.7- Radium's ability to glow in the dark |
Dangers of radium
 |
| Fig.8- Radium's health effect |
Radium is naturally occurred in the environment, but because of that we are always exposed to radium and due to the small amounts of radiation that it releases into the environment, it highly affects our health(Fig.8) .The radiation it gives off can kill living cells, which is why it is used to treat cancer. We, humans release radium into the environment by burning coal and other fuels.
Chemical Properties of radium
Radium combines with mostly non-metals, which includes oxygen, fluorine, chlorine, and nitrogen. “It also reacts with acids with the formation of hydrogen gas”.



